
Description
Valganciclovir hydrochloride (C14H22N6O4.Hcl; MWt=390.83) is the Hcl salt of the L-valyl ester of Ganciclovir, which is a synthetic 2′-deoxy-guanosine analogue used for the prevention and treatment of cytomegalovirus (CMV) infections in immunocompromised or immunosuppressed patients. Valganciclovir is the pro-drug for Ganciclovir. After oral administration, it is rapidly converted to Ganciclovir by intestinal and hepatic esterases.
Pharmacology
Following oral administration, Valganciclovir is rapidly hydrolysed to Ganciclovir by esterases in the intestinal and hepatic cells.
Absolute oral bioavailability is approximately 60%. When taken with food, a 900 mg dose of Valganciclovir is essentially equivalent to an IV Ganciclovir dose of 5 mg/kg. High fat food significantly increases the bioavailability and the peak serum level.
The time to maximum serum concentration (Cmax) is approximately 2 hours. The Cmax following the administration of 900 mg of Valganciclovir to adult patients with normal renal function is 5.6 and the serum level at 12 hours after the dose is about 1 µg/mL. In vitro, CMV resistance is defined as an IC50³1.5 µg/mL.
The plasma protein binding of Ganciclovir is 2% and its volume of distribution is approximately 0.7 L/kg. Liver transplant recipients attained similar exposures to Ganciclovir following the administration of 900 mg of Valganciclovir and 5 mg/kg IV Ganciclovir (42 vs 48 µg.hr/mL).
Valganciclovir is eliminated as Ganciclovir in the urine via glomerular filtration and active tubular secretion. The renal clearance of Ganciclovir is approximately 3 mL/min/kg.
The elimination half-life of Ganciclovir is about 4 hrs in patients with a creatinine clearance (ClCr) >75 mL/min and approximately 24 hrs in patients with CLcr between 10 and 20 mL/min.
Indications And Usage
Adult Patients:
- For the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS).
- For the prevention of CMV disease in kidney, heart and kidney-pancreas transplant patients at high risk.
Pediatric Patients:
For the prevention of CMV disease in kidney transplant patients (4 months to 16 years of age) and heart transplant patients (1 month to 16 years of age) at high risk.
Dosage And Administration:
VALSTEAD tablets should be taken with food.
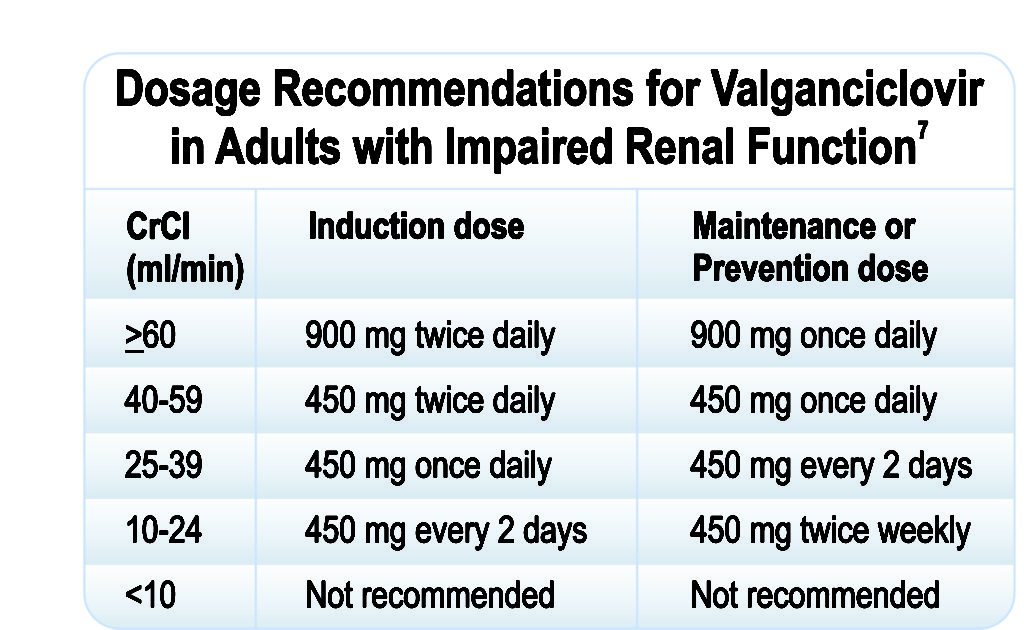
Dosage Recommendations for Adult Patients with Impaired Renal Function
Contraindications
Hypersensitivity to Valganciclovir or Ganciclovir
Valganciclovir is contraindicated in patients with:
Absolute neutrophil count < 500 cells/µL.
Platelet count < 25,000 platelets/µL.
Hemoglobin concentration < 8 grams/dL.
Warnings and Precautions
- Hematologic toxicity: Severe leukopenia, neutropenia, anaemia, thrombocytopenia, pancytopenia, bone marrow depression and aplastic anaemia have occurred with the use of Valganciclovir or Ganciclovir. Avoid VALSTEAD if absolute neutrophil count is less than 500 cells/μL, platelet count is less than 25,000/μL or haemoglobin is less than 8 g/dL. Use with caution in pre-existing cytopenias and when receiving myelosuppressive drugs or irradiation. Monitor with frequent testing of platelet and complete blood counts.
- Impairment of fertility: Based on animal studies, VALSTEAD may cause temporary or permanent inhibition of spermatogenesis.
- Foetal toxicity: Based on animal studies, VALSTEAD may cause foetal harm. Females of reproductive potential should use effective contraception during and following treatment and males should practice barrier contraception during and following the treatment.
- Mutagenicity and carcinogenicity: Based on animal studies, VALSTEAD is potentially mutagenic and carcinogenic.
- Acute renal failure: Acute renal failure may occur in elderly patients (with or without reduced renal function), patients who receive concomitant nephrotoxic drugs or inadequately hydrated patients. Use with caution in elderly patients or those taking nephrotoxic drugs, reduce dosage in patients with renal impairment and monitor renal function.
Adverse Reactions
- Adult patients: The most common adverse events and laboratory abnormalities are diarrhoea, pyrexia, nausea, tremor, neutropenia, anaemia, graft rejection, thrombocytopenia and vomiting.
- Pediatric patients: The most common adverse events and laboratory abnormalities are diarrhoea, pyrexia, hypertension, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia and headache.
Drug Interactions
- Zidovudine: Potential to cause neutropenia and anaemia. Monitor with frequent tests of white blood cell counts with differential and haemoglobin levels.
- Probenecid: May increase Ganciclovir levels. Monitor for evidence of Ganciclovir toxicity.
- Mycophenolate Mofetil (MMF): May increase Ganciclovir concentrations and levels of MMF metabolites in patients with renal impairment. Monitor for Ganciclovir and MMF toxicity.
- Didanosine: May increase didanosine concentrations. Monitor for didanosine toxicity.
Use In Specific Populations
Lactation: Breastfeeding is not recommended with use of VALSTEAD.
Presentation
4 tablets packed in a blister strip, 15 such blister strips packed in a carton.
