
DESCRIPTION:
ProCotri (sulfamethoxazole and trimethoprim) is a synthetic antibacterial combination of 800 mg sulfamethoxazole and 160 mg trimethoprim available in a tablet form.
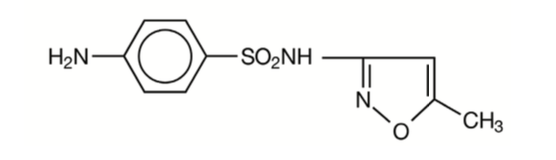
Sulfamethoxazole is N¹-(5-methyl-3-isoxazolyl) sulfanilamide; the molecular formula is C¹⁰H¹¹N³O³S. It is an almost white, odourless, tasteless compound with a molecular weight of 253.28 and the following structural formula:
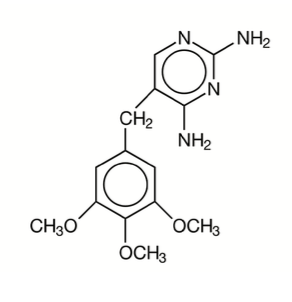
Trimethoprim is 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine; the molecular formula is C¹⁴H¹⁸N⁴O³. It is a white to light yellow, odourless, bitter compound with a molecular weight of 290.3 and the following structural formula:
COMPOSITION
Each uncoated tablet contains:
Trimethoprim I.P. 160 mg
Sulfamethoxazole I.P. 800 mg
Excipients q.s.
DOSAGE FORM
Tablet
CLINICAL PHARMACOLOGY
ProCotri is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound and metabolised forms; sulfamethoxazole also exists as the conjugated form. Sulfamethoxazole is metabolised in humans to at least 5 metabolites: the N⁴-acetyl-, N⁴-hydroxy-, 5-methylhydroxy-, N⁴-acetyl-5-methyl hydroxy – sulfamethoxazole metabolites, and an N – glucuronide conjugate. The formulation of N⁴ -hydroxy metabolite is mediated via CYP2C9.
Trimethoprim is metabolised in vitro to 11 different metabolites, of which, five are glutathione adducts and six are oxidative metabolites, including the major metabolites, 1- and 3-oxides and the 3- and 4-hydroxy derivatives.
The free forms of sulfamethoxazole and trimethoprim are considered to be the therapeutically active forms.
In vitro studies suggest that trimethoprim is a substrate of P-glycoprotein, OCT1 and OCT2, and that sulfamethoxazole is not a substrate of P-glycoprotein.
Approximately 70% of sulfamethoxazole and 44% of trimethoprim are bound to plasma proteins. The presence of 10 mg percent sulfamethoxazole in plasma decreases the protein binding of trimethoprim by an insignificant degree; trimethoprim does not influence the protein binding of sulfamethoxazole.
Peak blood levels for the individual components occur 1 to 4 hours after oral administration. The mean serum half-lives of sulfamethoxazole and trimethoprim are 10 and 8 to 10 hours, respectively. However, patients with severely impaired renal function exhibit an increase in the half-lives of both components, requiring dosage regimen adjustment (see DOSAGE AND ADMINISTRATION section). Detectable amounts of sulfamethoxazole and trimethoprim are present in the blood 24 hours after drug administration. During the administration of 800 mg sulfamethoxazole and 160 mg trimethoprim b.i.d., the mean steady-state plasma concentration of trimethoprim was 1.72 μg/mL. The steady-state mean plasma levels of free and total sulfamethoxazole were 57.4 μg/mL and 68.0 μg/mL, respectively. These steady-state levels were achieved after three days of drug administration.1 Excretion of sulfamethoxazole and trimethoprim is primarily by the kidneys through both glomerular filtration and tubular secretion. Urine concentrations of both sulfamethoxazole and trimethoprim are considerably higher than the concentrations in the blood. The average percentage of the dose recovered in urine from 0 to 72 hours after a single oral dose of sulfamethoxazole and trimethoprim is 84.5% for total sulfonamide and 66.8% for free trimethoprim. Thirty percent of the total sulfonamide is excreted as free sulfamethoxazole, with the remaining as N⁴ -acetylated metabolite.² When administered together as sulfamethoxazole and trimethoprim, neither sulfamethoxazole nor trimethoprim affects the urinary excretion pattern of the other.
Both sulfamethoxazole and trimethoprim distribute to sputum, vaginal fluid and middle ear fluid; trimethoprim also distributes to bronchial secretion, and both pass the placental barrier and are excreted in human milk.
Geriatric Pharmacokinetics: The pharmacokinetics of sulfamethoxazole 800 mg and trimethoprim 160 mg were studied in 6 geriatric subjects (mean age: 78.6 years) and 6 young healthy subjects (mean age: 29.3 years) using a non-US approved formulation. Pharmacokinetic values for sulfamethoxazole in geriatric subjects were similar to those observed in young adult subjects. The mean renal clearance of trimethoprim was significantly lower in geriatric subjects compared with young adult subjects (19 mL/h/kg vs. 55 mL/h/kg). However, after normalising by body weight, the apparent total body clearance of trimethoprim was on average 19% lower in geriatric subjects compared with young adult subjects.³
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ProCotri (sulfamethoxazole and trimethoprim) tablets and other antibacterial drugs, ProCotri (sulfamethoxazole and trimethoprim) tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Pneumocystis jiroveci Pneumonia: For the treatment of documented Pneumocystis jiroveci pneumonia and for prophylaxis against P. jiroveci pneumonia in individuals who are immunosuppressed and considered to be at an increased risk of developing P. jiroveci pneumonia.
Urinary Tract Infections: For the treatment of urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis and Proteus vulgaris. It is recommended that initial episodes of uncomplicated urinary tract infections be treated with a single effective antibacterial agent rather than the combination.
Acute Otitis Media: For the treatment of acute otitis media in pediatric patients due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of other antimicrobial agents.Till date, there are limited data on the safety of repeated use of ProCotri in pediatric patients under two years of age. ProCotri is not indicated for prophylactic or prolonged administration in otitis media at any age.
Acute Exacerbations of Chronic Bronchitis in Adults: For the treatment of acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when a physician deems that ProCotri could offer some advantage over the use of a single antimicrobial agent.
Shigellosis: For the treatment of enteritis caused by susceptible strains of Shigella flexneri and Shigella sonnei when antibacterial therapy is indicated.
Traveler’s Diarrhoea in Adults: For the treatment of traveler’s diarrhoea due to susceptible strains of enterotoxigenic E. coli.
CONTRAINDICATIONS
ProCotri is contraindicated in patients with a known hypersensitivity to trimethoprim or sulphonamides, in patients with a history of drug induced immune thrombocytopenia with use of trimethoprim and/or sulphonamides, and in patients with documented megaloblastic anaemia due to folate deficiency.
ProCotri is contraindicated in pediatric patients less than 2 months of age. ProCotri is also contraindicated in patients with marked hepatic damage or with severe renal insufficiency when renal function status cannot be monitored.
WARNINGS
Embryofetal Toxicity
Some epidemiologic studies suggest that exposure to sulfamethoxazole/trimethoprim during pregnancy may be associated with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular malformations, urinary tract defects, oral clefts, and club foot.
Hypersensitivity and Other Fatal Reactions
Sulfonamides, including sulfonamide-containing products such as sulfamethoxazole/trimethoprim, should be discontinued at the first appearance of skin rash or any sign of adverse reaction. Cough, shortness of breath, and pulmonary infiltrate are hypersensitivity reactions of the respiratory tract that have been reported in association with sulfonamide treatment.
Thrombocytopenia
Sulfamethoxazole/trimethoprim-induced thrombocytopenia may be an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life-threatening have been reported.Thrombocytopenia usually resolves within a week upon discontinuation of sulfamethoxazole/trimethoprim.
Clostridium difficile associated diarrhoea
Clostridium difficile-associated diarrhoea (CDAD) has been reported with the use of nearly all antibacterial agents, including ProCotri, and may range in severity from mild diarrhoea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to the overgrowth of C. difficile.
Streptococcal Infections and Rheumatic Fever
The sulfonamides should not be used for the treatment of group A β-hemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever.
PRECAUTIONS
Development of drug-resistant bacteria
Prescribing ProCotri (sulfamethoxazole and trimethoprim) in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Folate deficiency
ProCotri should be given with caution to patients with impaired renal or hepatic function, to those with possible folate deficiency(e.g., the elderly, chronic alcoholics, patients receiving anticonvulsant therapy, patients with malabsorption syndrome, and patients in malnutrition states) and those with severe allergies or bronchial asthma.
Hypoglycemia
Cases of hypoglycemia in non-diabetic patients treated with ProCotri are seen rarely, usually occurring after a few days of therapy. Patients with renal dysfunction, liver disease, malnutrition or those receiving high doses of ProCotri are particularly at risk.
Electrolyte Abnormalities
High dosage of trimethoprim, as used in patients with P. jiroveci pneumonia, induces a progressive but reversible increase of serum potassium concentrations in a substantial number of patients. Even treatment with recommended doses may cause hyperkalemia when trimethoprim is administered to patients with underlying disorders of potassium metabolism, with renal insufficiency, or if drugs known to induce hyperkalemia are given concomitantly. Close monitoring of serum potassium is warranted in these patients.
Severe and symptomatic hyponatremia can occur in patients receiving ProCotri, particularly for the treatment of P. jiroveci pneumonia. Evaluation for hyponatremia and appropriate correction is necessary for symptomatic patients to prevent life-threatening complications.
DRUG-DRUG INTERACTIONS
Potential for ProCotri to Affect Other Drugs
Trimethoprim is an inhibitor of CYP2C8 as well as OCT2 transporter. Sulfamethoxazole is an inhibitor of CYP2C9. Caution is recommended when Bactrim is co-administered with drugs that are substrates of CYP2C8 and 2C9 or OCT2.
In elderly patients concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with or without purpura has been reported.
Occasional reports suggest that patients receiving pyrimethamine as malaria prophylaxis in doses exceeding 25 mg weekly may develop megaloblastic anaemia if ProCotri is prescribed.
It has been reported that ProCotri may prolong the prothrombin time in patients who are receiving the anticoagulant warfarin (a CYP2C9 substrate). This interaction should be kept in mind when ProCotri is given to patients already on anticoagulant therapy, and the coagulation time should be reassessed.
DOSAGE & ADMINISTRATION
ProCotri is contraindicated in pediatric patients less than 2 months of age.
Pneumocystis Jiroveci Pneumonia
Treatment:
Adults And Children:
The recommended dosage for treatment of patients with documented Pneumocystis jiroveci pneumonia is 75 to 100 mg/kg sulfamethoxazole and 15 to 20 mg/kg trimethoprim per 24 hours given in equally divided doses every 6 hours for 14 to 21 days.4
Prophylaxis
Adults: The recommended dosage for prophylaxis in adults is 1 ProCotri tablet daily.⁵
Children: For children, the recommended dose is 750 mg/m²/day sulfamethoxazole with 150 mg/m²/day trimethoprim given orally in equally divided doses twice a day, on 3 consecutive days per week. The total daily dose should not exceed 1600 mg sulfamethoxazole and 320 mg trimethoprim.⁶
Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children:
Adults: The usual adult dosage in the treatment of urinary tract infections is 1 ProCotri tablet every 12 hours for 10 to 14 days. An identical daily dosage is used for 5 days in the treatment of shigellosis.
Children: The recommended dose for children with urinary tract infections or acute otitis media is 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in two divided doses every 12 hours for 10 days. An identical daily dosage is used for 5 days in the treatment of shigellosis.
Acute Exacerbations of Chronic Bronchitis in Adults:
The usual adult dosage in the treatment of acute exacerbations of chronic bronchitis is 1 ProCotri tablet every 12 hours for 14 days.
Traveler’s Diarrhoea In Adults:
For the treatment of traveler’s diarrhoea, the usual adult dosage is 1 ProCotri tablet every 12 hours for 5 days.
STABILITY AND STORAGE RECOMMENDATIONS
ProCotri tablets should be stored in a dry place at a temperature not exceeding 30°C. Protect from light and moisture.
REFERENCES:
1. KremersP,DuvivierJ,HeusghemC.PharmacokineticStudiesofCo-TrimoxazoleinManafterSingleand Repeated Doses. J Clin Pharmacol. FebMar 1974; 14:112–117.
2. KaplanSA,etal.PharmacokineticProfileofTrimethoprim-SulfamethoxazoleinMan.JInfectDis.Nov1973; 128(Suppl): S547–S555.
3. VaroquauxO,etal.Pharmacokineticsofthetrimethoprim-sulfamethoxazolecombinationintheelderly. Br J Clin Pharmacol. 1985;20:575–581.
4. MasurH.PreventionandtreatmentofPneumocystispneumonia.NEnglJMed.1992;327:1853–1880.
5. RecommendationsforprophylaxisagainstPneumocystiscariniipneumoniaforadultsandadolescentsinfected with human immunodeficiency virus. MMWR. 1992; 41(RR–4):1–11.
6. CDCGuidelinesforprophylaxisagainstPneumocystiscariniipneumoniaforchildreninfectedwithhuman immunodeficiency virus. MMWR. 1991; 40(RR–2):1–13.
